QuilliChew ER® tablets deliver
Eligible patients may PAY $25
Learn more about Savings & SupportTablets Can Be Swallowed Whole or Chewed
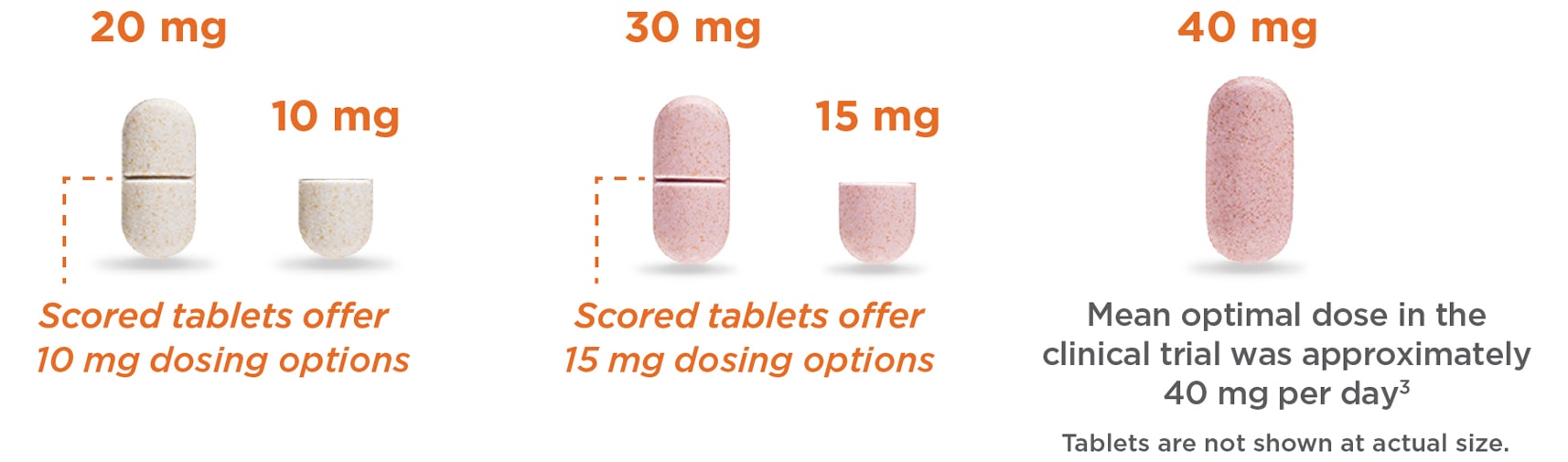
The only FDA-approved scored ER methylphenidate tablet
- 11 dosing options from 10 mg to 60 mg
- LiquiXR® technology allows titration in increments of 10 mg, 15 mg, or 20 mg to help balance efficacy and side effects
- Recommended starting dose of 20 mg given orally once daily in the morning2
- Dosage may be increased or decreased weekly in increments of 10 mg, 15 mg, or 20 mg2
- Daily dosage above 60 mg is not recommended2
QuilliChew ER Prescribing Guide

- Prior to treating patients with QuilliChew ER (methlyphenidate HCl), assess for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical examination) and risk for abuse, misuse, and addiction. Assess the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating QuilliChew ER2
- If paradoxical aggravation of symptoms or other adverse reactions occur, reduce dosage or discontinue the drug, if necessary. If improvement is not observed after appropriate dosage adjustment over a 1-month period, the drug should be discontinued2
If switching from other methylphenidate products to QuilliChew ER:
- Discontinue that treatment and titrate with QuilliChew ER using the above titration schedule
- Do not substitute for other methylphenidate products on a milligram-per-milligram basis because of different methylphenidate base compositions and differing pharmacokinetic profiles
Writing a prescription for QuilliChew ER
Example prescription

When prescribing multiple dosage strengths of QuilliChew ER with different NDCs when 1 dosage strength could be used to fill the prescription, it is important to remember:
- This may result in additional out-of-pocket or co-pay costs for your patients
- Insurance may cover a prescription for multiple strengths within one 30-day time frame; however, this may require prior authorization or a call to your patient's insurance company
- You may also want to consider other factors, such as tablet burden, when writing a prescription
CNS, central nervous system; ER, extended-release; FDA, US Food and Drug Administration; NDC, National Drug Code.
Reference: 1. Abbas R, Childress AC, Nagraj P, Rolke R, Berry SA, Palumbo DR. Relative bioavailability of methylphenidate extended-release chewable tablets chewed versus swallowed whole. Clin Ther. 2018;40(5):733-740. 2. QuilliChew ER [package insert]. Tris Pharma, Inc., Monmouth Junction, NJ. 3. Wigal SB, Childress A, Berry SA, et al. Optimization of methylphenidate extended release chewable tablet dose in children with ADHD: open-label dose optimization in a laboratory classroom study. J Child Adolesc Psychopharmacol. 2018;28(5):314-321.


